A useful measure of how much a gas deviates from ideal gas behaviour is found in its compressibility factor. THE COMPRESSIBILITY FACTOR of gas is the ratio PV/nRT. An ideal gas, the compressibility factor is 1. For a real gas, however, the compressibility factor have values that are significantly different from 1. The principal conclusion is that all gases behave ideally at low pressures but deviations set in at high pressures. At high pressures, compressibility factors are greater than 1.
TO SUMMARIZE:
- Gases tend to behave ideally at high temperatures and low pressures
- Gases tend to behave nonideally at low temperatures and high pressures
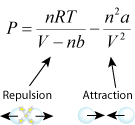
Pressure can be defined with the rearrangement of the equation as:

No comments:
Post a Comment